SERVICE

We will support your application for PMDA in Japan.
・IMPD(Investigational Medicinal Product Dossier)
・eCTD (electronic Common Technical Document)
・AFM(Accreditation of Foreign Manufacturers)
・DMF(Drug Master File)
Contents and flow of support
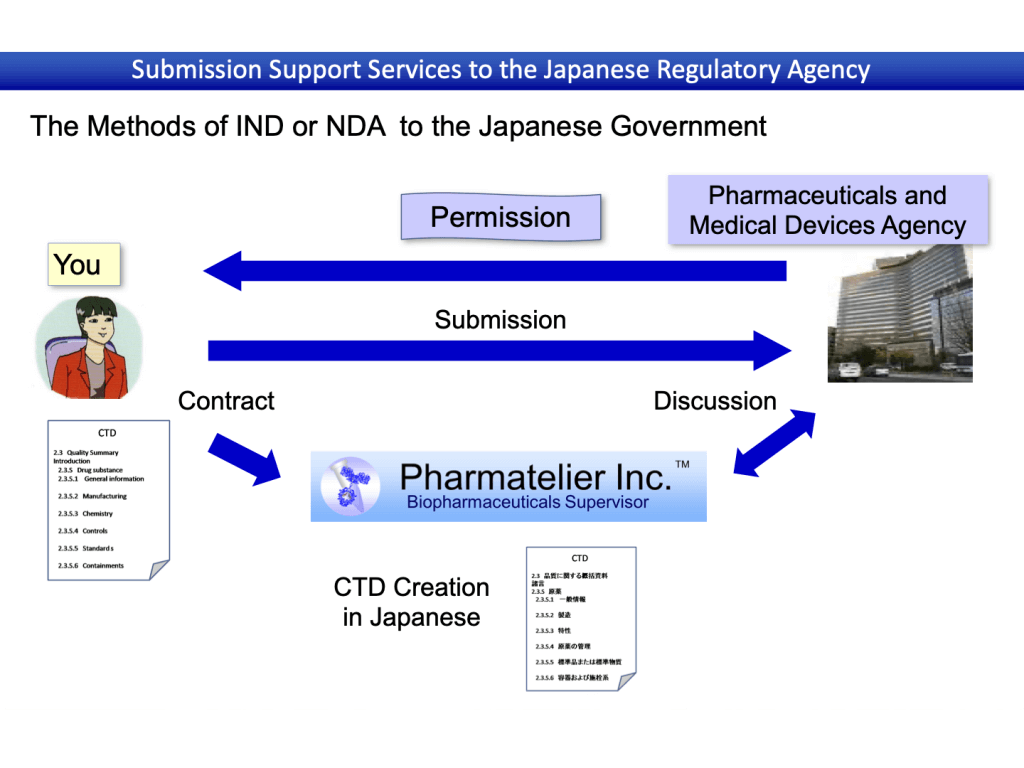
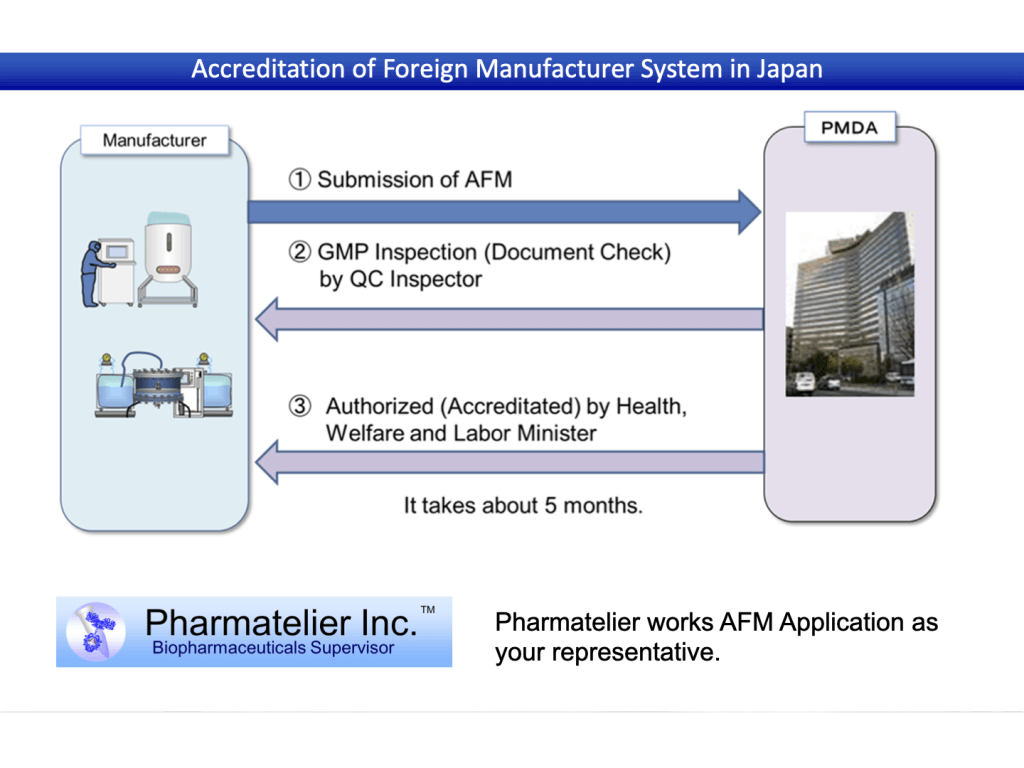
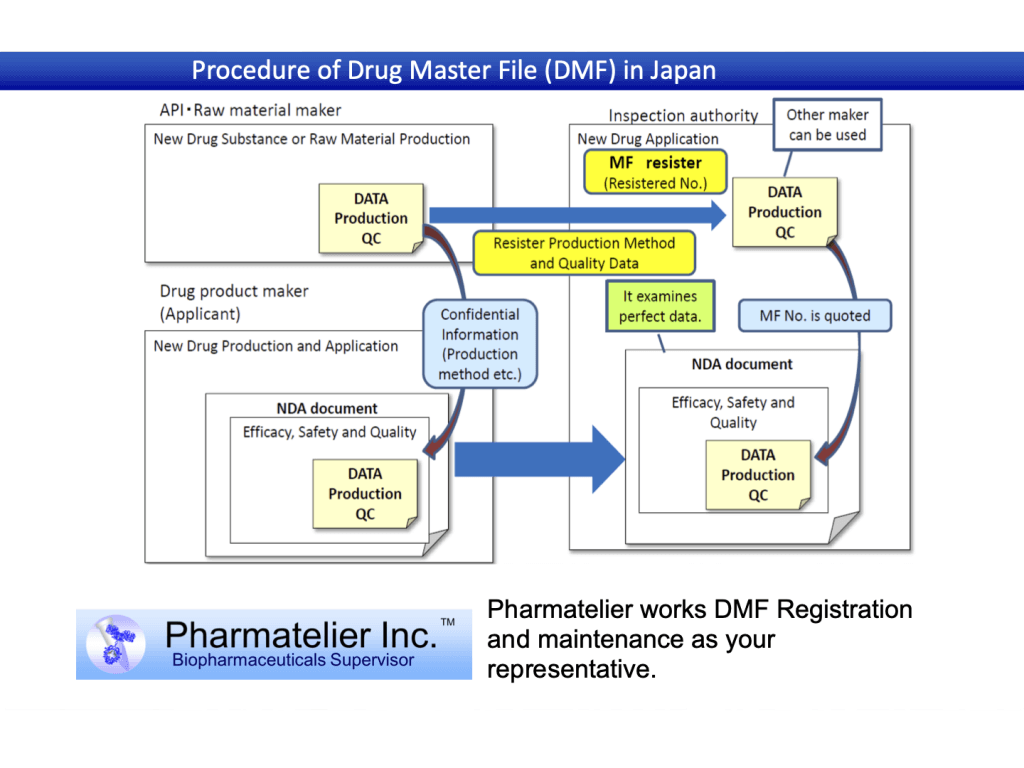
Application support results
・Preparation of US Drug Master File for new biotech products (translation of Japanese into English)
・Support for conducting clinical trials of biosimilars in Japan Consulting with PMDA on quality consultation measures
・Research on introduction of biosimilars from overseas companies
・Consultation on quality consultation with PMDA
・Master File of Biopharmaceuticals (MF) → 57 registered products (as of April 2023)
・Support for preparation of biosimilar monoclonal antibody application in Japan → Approval for manufacturing and marketing in Japan
・Support for preparation of application form for new monoclonal antibody in Japan → Manufacturing and marketing approval in Japan
・Biologics, pharmaceuticals, sterile drugs, regenerative medical products (North America, Europe, Korea, Taiwan,China)
・Support to preparation of approval application for COVID19 vaccine manufacturing and marketing authorization(CTD)
Consult with Pharmatelier about drug development
We will provide you with accurate and speedy support to solve various issues that may arise during the development and application process. Please feel free to contact us.
Consult with Pharmatelier about drug development
Consult with Pharmatelier
about drug development
We will provide you with accurate and speedy support to solve various issues that may arise during
the development and application process. Please feel free to contact us.
